Gibb's Free Energy Change in Terms of Gas Constant, Absolute Temperature, and Equilibrium Constant |
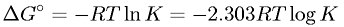 |
LaTeX Code:
\Delta G^\circ = - RT\ln K = - 2.303RT\log K
|
| MathType 5.0 Code:
% MathType!MTEF!2!1!+-
% feaafaart1ev1aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLn
% hiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqr1ngB
% PrgifHhDYfgasaacH8YjY-vipgYlh9vqqj-hEeeu0xXdbba9frFj0-
% OqFfea0dXdd9vqai-hGuQ8kuc9pgc9q8qqaq-dir-f0-yqaiVgFr0x
% fr-xfr-xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq
% Kaam4raiabgclaWkabg2da9iabgkHiTiaadkfacaWGubGaciiBaiaa
% c6gacaWGlbGaeyypa0JaeyOeI0IaaGOmaiaac6cacaaIZaGaaGimai
% aaiodacaWGsbGaamivaiGacYgacaGGVbGaai4zaiaadUeaaaa!4CEF!
|
|
Pasting the above "text" into MathType 5.0 will enter the above equation and make it possible to edit, save, and use in applications such as MS Word.
|


